Water Usage and Pollution
Water Usage
Unfortunately, on top of the water requirements to sustain life, we use our water for waste disposal, recreation, transportation, farming, and industrial purposes. In fact, between the years 1997-2003, the world’s per capita water usage worked out to about 1200 cubic meters per person! Rivers and streams certainly play an important role in our water usage for those communities that happen to be close to them; in the case of St. Louis, Missouri, we are heavily dependent on the Missouri River for our water supply. Recharge of aboveground water supplies is an even more difficult issue, since flood mitigation approaches such as channelization of waterways can actually increase the incidence of flash flooding and decrease the steady-state water level, since it decreases water infiltration and, consequently, the water table.
In many cases and in increasingly dry environments, that water usage is at the cost of harming the ecosystems that rely on that water. Balancing all our water usage requirements while not adversely impacting biodiversity is a significant challenge in the modern world.
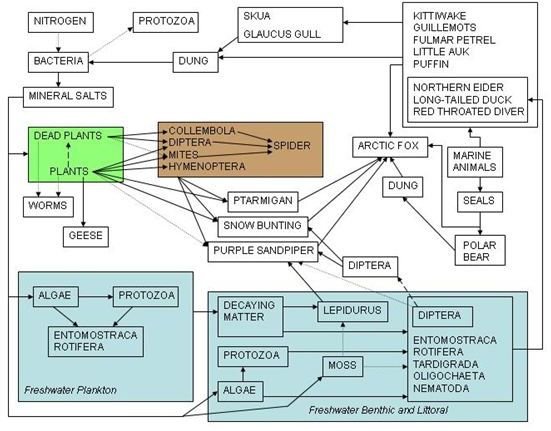
Trophic levels
With water serving so many different purposes, it seems obvious that pollution of that same water would be a major concern, but why (and how) exactly is that the case?
One significant issue, connected with food chains or “trophic levels”, is that of concentration of toxins; even though the concentration levels of a given pollutant may be low enough to avoid harming producers or low-level consumers, those compounds are concentrated by the action of the food chain itself. Even though the levels of a particular toxin (say, mercury) might be 1000 times below a lethal dose in a given plankton, if a fish eats a thousand of the plankton (and the chemicals remain in its system), then the concentration becomes high enough to harm humans. Since every living thing needs water, pollution of water can have far greater effects than pollution of an equivalent amount of land. Water also gives pollution “legs” and allows it to spread more rapidly, and a toxin that might not harm one organism could cripple or kill others in the exact same concentrations. This is especially true in the case of compounds like mercury that aren’t flushed out of an organism’s system.
Natural water filtration
Another is the amount of time necessary to clean polluted waters via natural means. Vegetation, soil, and porous rock formations serve as a filter, removing debris and chemicals from the water. As we increase the amount of impervious surfaces (asphalt, concrete, etc.) in our cities and elsewhere, pollutants run off before they can be filtered out of the water naturally. Such actions increase the water treatment cost to society while simultaneously decreasing the environment’s ability to cope with extreme weather conditions and adding biological stressors to the system. Even when filtration is possible, it may take many human lifetimes for groundwater to return to normal after contamination.
This post is part of the series: Water, Water…
Water is absolutely critical to the existence of life on Earth, and, for the needs of a significant fraction of flora and fauna, that water must be in the form of freshwater. Where does it come from, what affects it, what issues surround it, and how does it affect organisms? Find out here!