Bowen's Reaction Series and How Igneous Rocks Form
What is Bowen’s Reaction Series?
Igneous rocks are rocks that solidified from magma, or molten rock. There are six types of igneous rocks, as classified according to the types and average grain size of their constituent minerals. The mineral composition of a rock depends on the chemical composition of the magma it formed from - especially the percentage of silicon. A magma composed of under 50% silicon is called mafic, short for “magnesium-ferric” for the higher proportions of magnesium and iron. Over 65% silicon is a felsic magma, which means it will form lots of feldspars.
Mafic minerals have a higher crystallization temperature than felsic minerals - which means that as the magma cools, they form first. The order that minerals form - from mafic to felsic - is called Bowen’s reaction series. There are two groups: the ferromagnesians and plagioclase.
The Ferromagnesian Minerals of the Discontinuous Branch
The ferromagnesian minerals contain magnesium and iron. In order from mafic to felsic, these are olivine, pyroxenes such as augite, amphiboles such as hornblende, and biotite. Olivine looks like rounded olive-green grains. The others are greenish-black.
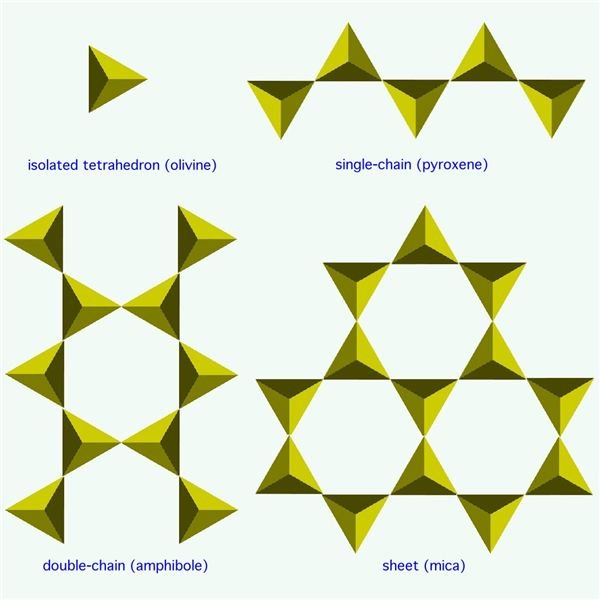
Olivine crystallizes first. It forms with its silicon arranged in isolated tetrahedrons of SiO4. With a general chemical formula of (Mg,Fe)2SiO4 (ranging from Mg2SiO4 (forsterite) to MgFeSiO4 to Fe2SiO4 (fayalite)), olivine has proportions of one part silicon to two parts iron or magnesium (a 1:2 ratio), which means it depletes the magma of iron and magnesium and leaves the remaining liquid with proportionately more silicon.
When the magma cools to the temperature for pyroxene to crystallize, some of the olivine reforms into pyroxene, which has the form of tetrahedrons in single chains (for each silicon atom, two oxygen atoms shared with another silicon). The general chemical formula is MgSiO3 (enstatite) or FeSiO3 (ferrosilite), giving it a 1:1 ratio of silicon to magnesium and iron. (Or CaSiO3 (wollastonite), or any combination of Fe, Mg, and Ca with Si2O6.) If there’s still magma left over, it has even more silicon in it than before.



As the magma continues to cool, amphiboles begin to crystallize, and some of the pyroxene reforms into amphibole. This has the tetrahedrons in double chains (a third oxygen shared with a silicon atom of the other chain). The chemical formula gets rather complicated, as a number of elements other than iron and magnesium can get incorporated, but the silicon part is basically two chains of Si4O11 (or a double chain of Si8O22).
The next thing to form is biotite, a type of mica. The tetrahedrons are arranged in sheets (three oxygens shared per silicon in a hexagonal pattern). Biotite uses up the last of the iron and magnesium in the magma. Usually by this point, all of the initial olivine has converted down into other things and there’s none left in the final rock.
Discontinuous vs. Continuous Branches of Bowen’s Reaction Series
The ferromagnesian minerals are called the discontinuous branch of Bowen’s reaction series because stuff happens at discrete intervals - whenever the magma cools down to the melting point of the next mineral in the series. In the continuous branch, changes occur gradationally across the entire temperature range for a single mineral.
Plagioclase Feldspar on the Continuous Branch
Plagioclase, a type of feldspar, is a dull, off-white color. Plagioclase can be thought of as a chemical composition range of the same mineral, with anorthite (CaAl2Si2O8) at the higher-temperature mafic endpoint and albite (NaAlSi3O8) at the lower-temperature felsic endpoint. As the magma gradually cools, plagioclase starts out forming with calcium, then gradually incorporates more and more sodium and runs out of calcium, until by the end it’s basically all sodium. The midpoint - where the ratio of calcium to sodium is 1:1 - happens at around the same time that pyroxene is turning into amphibole.



Orthoclase, Muscovite, and Quartz
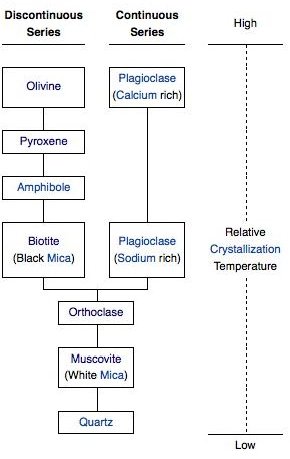
If there’s still liquid magma left after the two branches have run out of iron, magnesium, calcium, and sodium, what’s left over is mostly potassium, aluminum, and lots of silicon.
The potassium and aluminum get used up to make orthoclase, which is potassium feldspar (KAlSi3O8). If the magma contains a lot of water, muscovite will also form ( KAl2(AlSi3O10)(OH)2 ). Orthoclase is a salmon pink color. Muscovite is a mica like biotite, but translucent rather than black.
Finally, any silicon left over after all the other chemical elements have been used up becomes quartz, which is just straight silica, or SiO2. In quartz, the silicon atoms share all four of their oxygens with other silicon atoms in a 3D framework structure. Quartz marks the extreme felsic end of the mafic-felsic range.
Mafic and Felsic Rocks
The formation of the final type of igneous rock depends on how far down the series it gets before the magma has completely solidified. A low percentage of silicon results in mafic rocks like basalt and gabbro, which have minerals like olivine, augite, and calcium-rich plagioclase. High silicon results in felsic rocks like granite and rhyolite, rich in hornblende, sodium-rich plagioclase, orthoclase, biotite, and quartz.
The Role of Bowen’s Reaction Series in Sedimentary Processes
During chemical weathering, most of the minerals in igneous rocks decompose into the minerals found in clay. (Quartz becomes sand.) The minerals that crystallize first, at the highest temperature, are the least stable at typical temperatures and pressures on Earth’s surface. They therefore weather the fastest. Chemical weathering rates for the different minerals follow Bowen’s reaction series in the same order, from mafic to felsic.
References and Photo Credits
Plummer, Charles C. and McGeary, David. Physical Geology 5th ed. 1991.
Dana, James D. Manual of Mineralogy 20th ed. Revised by Cornelis Klein and Cornelius S. Hurlbut Jr. 1985.
Olivine image by V. Smith, biotite image by Didier Descouens, used under CC-A-SA-3.0 unported license
Feldspar image by Dave Dyet, public domain
Muscovite image by Rob Lavinsky, used under CC-A-SA-3.0 license
Augite and quartz images by U.S. Geological Survey, public domain
Bowen’s reaction series diagram from Wikipedia
Silicate structure diagram by article author
This post is part of the series: The Basics About Igneous Rocks and Minerals
Learn how igneous rocks are classified, and how they form from magma, in this series of articles.