How do Hydrogen Fuel Cells Work?
Introduction to Hydrogen Fuel Cells
A Hydrogen fuel cell converts chemical energy into electrical energy, through the supply of hydrogen and oxygen gasses which react in the presence of catalysts on anode and cathode plates.
The concept of a fuel cell was recognized a hundred and fifty years ago, but it was not until the 1920’s that they were developed into an early form of what we have today.
Energy experts cite fuel cells as too expensive and bulky for use in automobiles, however throughout the world today there are many vehicles and buildings using fuel cell as as a means of power.
This is an article on hydrogen fuel cells where we will examine a few of the different types of cells, and we begin with a brief history of fuel cells.
History of Fuel Cells
Fuel cells were first conceived in 1839 when a Welsh scientist used hydrogen and oxygen gases combined with an electrolyte to produce a small electric current. However it was not until eighty years later that German technology ushered in the fuel cell as we know it today.
In 1955 Francis Bacon, a British engineer and an advocate and expert of fuel cells technology was successful in linking a bank of fuel cells to produce 5kW of power.
NASA decided to use fuel cells to provide electrical power for their Gemini and Apollo spacecraft. This led to a spate of research and development projects in the 1960’s with NASA funding a great number of them. This era proved to be turning point in hydrogen fuel cell technology, which has been ongoing up to the present day. An advantage of fuel cells is that the Space Shuttle uses them for electrical power, with the residual H2O being used as drinking water.
Regrettably, the Obama administration is withdrawing funding for fuel cell development, stating that other means of renewable energy sources such as bio-fuels reflect a more realistic and viable alternative to fossil fuels.
However numerous vehicle manufacturers are continuing to develop the fuel cells from the NASA designs. Daimler, Nissan, and Honda have introduced buses and fuel cell cars which can be supplied with hydrogen at selective fuel outlets.
Types of Hydrogen Fuel Cells
There are numerous types of hydrogen fuel cells, some of the current ones in use or being developed are listed below;
- **Polymer Exchange Membrane Fuel Cell (**PEMFC) – in use today, very suitable for powering vehicles
- Phosphoric Acid Fuel Cell (PAFC) – many in use today generating power to houses and public buildings
- Solid Oxide Fuel Cell (SOFC) – large scale power generation (Research and development ongoing)
- Alkaline Fuel Cell (AFC) – used in spacecraft and military applications
- Molten Carbonate Fuel Cell (MCFC) – large scale power generation from both the steam by product and electricity produced (Research and development ongoing)
The Polymer Exchange Membrane Fuel Cell is the top candidate for vehicle propulsion, so we shall have a closer look at it in the next section.
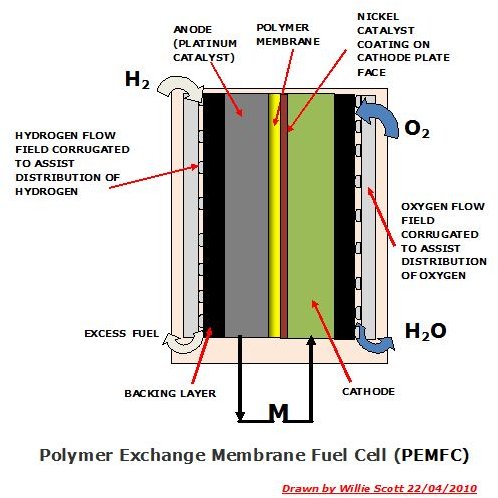
Polymer Exchange Membrane Fuel Cell (PEMFC)
A Polymer Exchange Membrane Fuel Cell consists of a rectangular cell containing an anode and cathode plate, being grooved to assist distribution of the fuel and oxidant in the their respective flow fields. The anode is fabricated from compressed platinum powder, the cathode having just a thin coating of nickel on the inner side of the plate, the platinum and nickel acting as catalysts.
In between the plates is a polymer membrane that allows positive hydrogen ions to pass through to the cathode plate, but not the negative electrons. Pressurised hydrogen gas is fed into the anode plate distribution field where, due to the platinum, the H2 splits into two positive ions and two negative electrons. As negative hydrogen electrons cannot pass through the membrane these are forced to travel from the anode plate through an external electrical circuit, driving an electrical component en route before returning to the cell through the cathode plate.
Meanwhile, oxygen in the form of ambient air is fed into the cathode plate distribution field, and on contact with the nickel catalyst, converts the oxygen atoms into two highly charged negative atoms. These attract the positive hydrogen ions through the membrane where they combine with oxygen atoms and the electrons returning from the electrical circuit to form H2O, which exits the cell as waste water.
The electrical output from a hydrogen fuel cell is quite small so to drive a car numerous cells are linked together in a system known as stacking.