Carbon Dioxide Capture Methods.
Introduction
With the continuous increase in the level of carbon dioxide in the atmosphere, the whole world is experiencing the ill effects of greenhouse gases. The release of carbon dioxide, the main culprit behind the global warming phenomenon, needs to be controlled. People around the world are already seeking for ways to reduce the amount of carbon dioxide that is being added to the atmosphere each day. Some of the most commonly sought after ways of them are finding new renewable sources of fuel and increasing the green cover of the world. Though significant steps, both will take a considerable amount of time before global warming is stopped, leading to an undesirable and uncontrolled condition. However, the need of the situation demands a fast and drastic reduction in carbon dioxide that is already present in the atmosphere.
Green plants are the one and only medium which helps in scrubbing carbon dioxide from the atmosphere. However, the amount of carbon dioxide that comes from automobiles is way too high to be absorbed by the plants. Thus there is a need of an alternate system which sucks the atmospheric carbon dioxide out of the atmosphere quickly and efficiently.
Removing released carbon dioxide from the air is not a new concept. In fact many research facilities around the world have been working on this idea since the time the concept of carbon capture and storage (CCS) was first implemented in 2005. However, until now, there have been three main methods of absorbing and removing carbon dioxide from the atmosphere.
Capturing Airborne Carbon Dioxide
The following three methods, though still in the implementation process, are the most promising ones. However, it is to note that none of them have been able to put into practice because of one or another drawbacks.
The Spray Hangar Method
The spray hanger method is a process by which carbon dioxide can be captured from the ambient air using a special arrangement. This process uses sodium hydroxide solution to extract carbon dioxide from thin air. The apparatus consists of a chamber with a fan at one end, which pulls the air inside it. The nozzles attached at the top of the chamber spray sodium hydroxide over the incoming air. The nozzle spray creates a mist and the sodium hydroxide solution reacts with the carbon dioxide present in the air to produce sodium carbonate, which is collected from a drain provided at the bottom of the chamber. The drain takes sodium carbonate to a separate chamber wherein through a special chemical process, involving sodium carbonate and calcium hydroxide, sodium hydroxide is again procured. This reaction not only reproduces sodium hydroxide but also carbon dioxide. The carbon dioxide produces is separated, compressed, and stored.
The main advantage of this concept is that all the chemicals used in the process have already been tried and tested several times for the same purpose, increasing the probability of successfully capturing carbon dioxide from thin air. However, as carbon dioxide present in the air is very small in quantity, the surface area required by the chamber is very large. Moreover, the system utilizes a large amount of energy for the overall process, making it uneconomical.
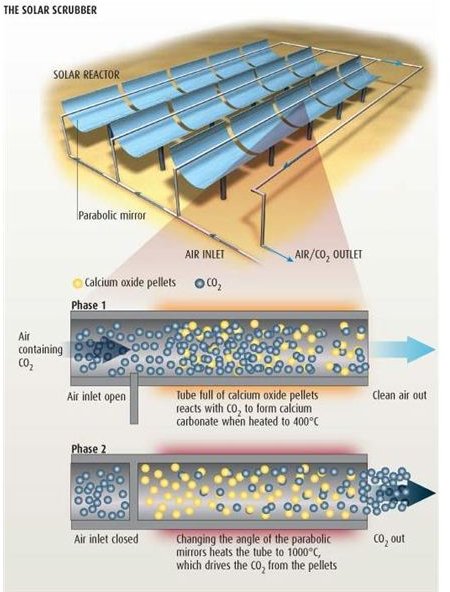
The Solar Scrubber Method
The second successful method for capturing carbon dioxide is that of a solar scrubber, which consists of transparent tubes filled with calcium oxide pellets. The tubes are heated with a heating source when atmospheric air mixed with steam is passed through the tubes. There are two heating modes used for this. First, a set of parabolic mirrors which concentrate the sun’s rays to heat up the tube, and second is an arc lamp.
When the inlet of the tube is open, air mixed with small amount of steam enters the tube. The light from the arc lamp heats the tube and its content up to 400 degree Celsius. At this temperature, the carbon dioxide from the air reacts with calcium oxide to form calcium carbonate. When the conversion is complete, the inlet is shut and the temperature inside the tube is increased to 800 degree Celsius, which boils off the carbon dioxide into pure gas. The pure carbon dioxide gas is then stored and the calcium oxide which remains at the end is again converted into pellets and reused for the process.
The main advantage of this process is that it can work thoroughly on a renewable source of energy. However, the setup requires enough parabolic mirrors and sunlight to raise the temperature.
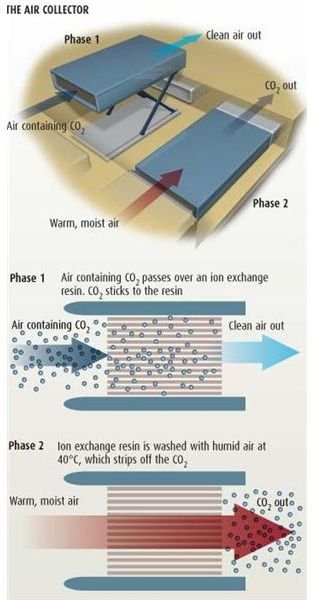
The Air Collector Method
The third method, the air collector, uses an ion exchange resin made from polymerization and filled with sodium hydroxide solution for capturing carbon dioxide from air. The polymerized resin is kept in an airtight chamber and the carbon dioxide filled air is passed through it. The main reason of using sodium hydroxide in the resin is that the sodium ions stick firmly with the polymers, but the hydroxide ions, which are loose, are later displaced by the carbon dioxide molecules. The carbon dioxide molecules then bind with sodium hydroxide and stick. The air released on the other side is clean and without any carbon dioxide.
Another advantage of the resin is that it changes is state when comes in contact with moisture. Thus, when warm moisture laden air (having an approximate temperature of 40 degree celsius) is passed through the resin the later chances its state, releasing the carbon dioxide bonded to its surface. The pure carbon dioxide released from the other end of the chamber is captured and stored.
The main advantage of this process is that there is no need of an external energy source. However, the resin used is highly expensive and difficult to make.