Learn How Crystals are Formed and About Processes for Growing Crystals
What is a Crystal
A crystal is a solid whose atoms and molecules are connected together in a very orderly arrangement. It is sometimes called a crystalline solid. A noncrystalline solid, on the other hand, is one whose atoms and molecules are connected together in a more haphazard manner. A simple, common crystalline material is table salt, sodium chloride (NaCl). Each molecule of

sodium chloride contains only two atoms, one of sodium (Na) and one of chloride (Cl).
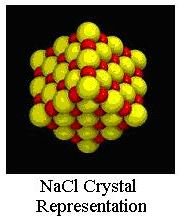
Sodium chloride is an ionic compound, made up of a positive sodium ion (Na+) and a negative chloride ion (Cl-). The Na+ and Cl- ions are attracted to each other, so the ion arrangement in the salt crystal is such that the sodium ions are surrounded by chloride ions and chloride ions are surrounded by sodium ions, as shown in the representation of a NaCl crystal at the upper left. This makes for rectangular salt crystals as shown in the picture of NaCl crystals at the right.
How Crystals are Formed
In very general terms, crystals are formed by either of two methods: i) cooling of a liquid until it solidifies (freezes) into a crystalline solid, or ii) evaporation of the solvent from a solution in which the solute is the compound being crystallized. The first method is illustrated by water freezing into ice crystals or formation of crystals within the earth long ago, when molten rock cooled. For the second method, the solvent is often water, as for example when the water is allowed to evaporate from a concentrated salt solution, leaving salt crystals as a residue.

The crystals found in the earth (minerals, diamonds, emeralds, or sapphires for example) were formed naturally as the earth cooled, and molten rock solidified under great pressure. The picture at the left shows some beautiful sapphire and diamond crystals formed by such processes. Another example is salt deposits near oceans and seas formed when water evaporated naturally from trapped seawater. Crystals can, however, be manmade. The process of crystallization is used to separate and purify compounds that can be crystallized from solution. This process of growing crystals is described in the next section.
Crystallization: Growing Crystals
Crystallization, as used to purify a solid compound, is called “recrystallization.” This purification process is started by dissolving an impure

solid compound in a solvent. It may be necessary to heat the solution to dissolve the solid or it may be soluble at room temperature. At any rate the crystallization process is started by slowly cooling the solution. As the solution cools, the solid compound will slowly form crystals that continue to grow as the solutions continues to cool. If the solution is cooled too rapidly, impurities may become trapped in the crystal structure, but if the crystallization takes place slowly enough, the growing crystals will be the pure compound of interest.
References and Image Credits
References for Further Information:
1. The Chemical Engineer’s Resource Page
2. CU Boulder Organic Chemistry Undergraduate Chemistry Courses
3. Ulrich, J. and Jones, M.J., Chemical Engineering and Chemical Process Technology -Heat and Mass Transfer Operations - Crystallization (PDF)
Image Credits:
NaCl Crystal Representation - The Science of Food
Salt (NaCl) Crystals - Flicker.com
Blue Sapphire and Diamonds - picasaweb.google.com
Laboratory Apparatus - Science Museum/Science and Society Picture