"What are the 5 Major Greenhouse Gases?" does have a Definitive Answer
Background on Greenhouse Gases
Greenhouse gases in the atmosphere are similar to the glass covering a greenhouse. That is, they allow short wavelength solar radiation to
pass through the atmosphere to reach the earth’s surface, but absorb the longer wavelength heat that is radiated back into the atmosphere from the earth. The concentration of greenhouse gases in the atmosphere thus has an effect on the average temperature at the surface of the earth. If the atmospheric concentration of greenhouse gases decreases over time, then more heat will escape through the atmosphere, and the average temperature at the earth’s surface will go down. If the greenhouse gas concentration in the atmosphere increases, however, less heat will escape to outer space and the average temperature at the earth’s surface will increase.
Based on a National Oceanic and Atmospheric Administration (NOAA) research report1, five major greenhouse gases, carbon dioxide, methane, nitrous oxide, CFC-12 and CFC-11, are responsible for 96% of the radiative forcing (greenhouse effect) since 1750.
Carbon Dioxide
Carbon dioxide (CO2) is one of the end products of the oxidation of organic matter. Thus carbon dioxide is given off due to combustion of any organic fuel (wood, natural gas, fuel oil, coal, etc.) Carbon dioxide is also given off from decomposition and decay of dead plant and animal matter. The decomposition/decay process is simply recycling back the carbon dioxide that was taken out of the atmosphere by photosynthesis to grow the plants (the organic carbon cycle). When fossil fuels (coal, natural gas, or petroleum based fuels) are burned, however, carbon dioxide that had been stored underground for millennia is released into the atmosphere.
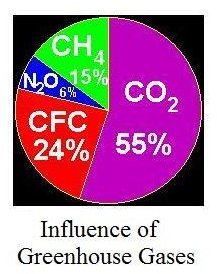
Some of the carbon dioxide from the atmosphere is taken up by plants through photosynthesis; some is absorbed by the ocean waters, and some is absorbed in soil. Currently however, we’re putting carbon dioxide into the atmosphere faster than it is coming out. Based on measurements that were initiated in the latter half of the 20th century, carbon dioxide levels in the atmosphere have increased from about 270 ppm before the beginning of the industrial revolution in the mid-1700s to about 380 ppm today.2 The diagram at the left shows the relative contribution of several gases to the greenhouse effect. As shown, carbon dioxide in the atmosphere is responsible for 55% of the greenhouse effect.
Methane
Methane (CH4) is the main component of natural gas, which is widely used as a fuel. The methane that we’re concerned about in the atmosphere, however, doesn’t come from burning natural gas (which puts carbon dioxide into the atmosphere, but not methane). The primary source of methane in the atmosphere is anaerobic decomposition of organic matter, for which methane is one of the end products. Some of the anaerobic processes (meaning in the absence of oxygen) that produce methane are in sanitary landfills, from concentrated waste organic matter (like livestock manure), from bogs and swamps, and (believe it on not) from termites. Methane is produced in anaerobic digesters in wastewater treatment plants, but in most cases it is either captured for use or burned in a flare.
Atmospheric methane is oxidized to carbon dioxide, or carbon monoxide and water, when it comes into contact with a strong oxidizing agent like hydroxyl radicals. Measurement of atmospheric methane concentrations began in the late 1970s, showing it to increase from 1.52 ppm in 1978 to about 1.77 ppm in 1990.2 It has remained approximately constant since then. This is only about 5% of the carbon dioxide concentration; however methane is approximately 25 times as effective as carbon dioxide in retaining heat in the atmosphere.3
Nitrous Oxide
Nitrous Oxide (N2O) is probably most widely known through its use as laughing gas, a mild anesthetic. The sources for its presence in the atmosphere are a variety of agricultural and industrial sources.4 Nitrous oxide is part of the natural nitrogen cycle as well; it is an intermediate in the denitrification of nitrate to nitrogen gas. A significant characteristic of nitrous oxide as a greenhouse gas is its longevity, with an average persistence in the atmosphere of 120 years. There hasn’t been nearly as much measurement of nitrous oxide concentrations in the atmosphere as there has been for carbon dioxide, or even for methane. The global average concentration of nitrous oxide in the atmosphere in 1998 was about 314 ppb, about one thousandth of the concentrations of atmospheric carbon dioxide. Even with this low concentration, nitrous oxide exerts a greenhouse effect, because it is approximately 300 times as effective as carbon dioxide in retaining heat in the atmosphere.3
CFC-12 and CFC-11
CFC-11 (CCl3F) and CFC-12 (CCl2F2) are the two chlorofluorocarbons (CFCs) that have the greatest greenhouse effect. CFCs do not occur naturally. They were first created in 1928, and saw significant use as cleaning solvents, aerosol propellants, and refrigerants. Based on the effect of CFCs in destroying stratospheric ozone, a very successful global effort has essentially halted their production, so that atmospheric levels are now remaining constant or decreasing. Atmospheric concentrations increased from zero before 1928 to current levels of about 240 ppt for CFC-11 and about 530 ppt for CFC-12. At parts per trillion, the concentrations of these two CFCs are less than atmospheric carbon dioxide concentrations by a factor of 106. They still have some greenhouse gas effect, because they are 5,000 to 10,000 times as effective as carbon dioxide in retaining heat in the atmosphere.3
Other Greenhouse Gases
Ozone, water vapor, and other CFCs and halofluorocarbons also exhibit greenhouse gas characteristics. Ozone in the troposphere is already regulated for health-related reasons, so it normally isn’t included in the inventory of greenhouse gases. Water vapor is actually the greenhouse gas that is present in the atmosphere in the greatest concentration; however its concentration is not believed to have been affected by industrialization and activities of mankind, so it is not regulated as a greenhouse gas. Other CFC and halofluorocarbon greenhouse gases are present in lower concentrations than CFC-11 and CFC-12.
References and Image Credits
1. https://www.esrl.noaa.gov/gmd/aggi/
2. https://www.ncdc.noaa.gov/oa/climate/gases.html
3. https://cdiac.ornl.gov/pns/current_ghg.html
4. https://www.epa.gov/nitrousoxide/scientific.html
First image: https://tonto.eia.doe.gov/energyexplained/index.cfm?page=environment_about_ghg
Second image: https://upload.wikimedia.org/wikipedia/commons/f/f0/Influence_of_greenhouse_gas.gif
