Basic Concepts of the Distillation of Alcohol
Distillation Process of Alcohol
In the distillation process of alcohol, a mixture of liquid is split into its contents by heating. In the distillation of water contaminated water is evaporated by heating and then it is condensed back to liquid leaving impurities behind. We see this process in nature when the sun heats up the lake water and causes it to evaporate, and the vapor is then condensed back to earth as pure water (rain). The process of distillation is widely used in medicinal products manufacturing and in the oil industry.
How to Produce Alcohol
One of the oldest methods is the fermentation of carbohydrates.
Sugar → Alcohol + CO2.
To trigger the above reaction more quickly, a catalyst called Zymase can be added. Fermentation is done in the absence of oxygen; otherwise it will burn the sugar into CO2 + Oxygen.
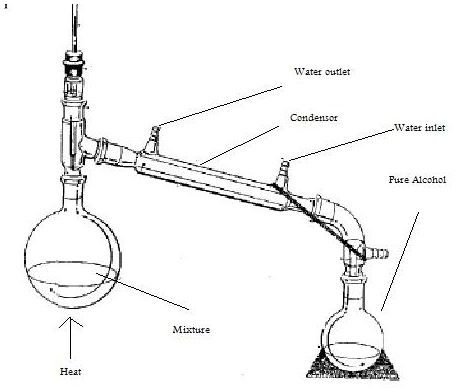
Alcohol distillation
Ethyl alcohol is used as alternative source of energy (fuel) for cooking and lighting (Lanterns).It is also widely used in medicines and lotions. In alcohol distillation, the alcoholic mixture is boiled at specific temperature to make the alcoholic contents evaporate which is then condensed back to liquid by process called condensation. Different contents (liquids) have different molecules structure, so their boiling point varies from one another. Water hasa boiling point of 100C while alcohol starts boiling at 78.5C.So on heating the mixture of pure alcohol and water we get alcoholic content first as vapors.
Alcohol Distillation process step by step
Required items: A distillation apparatus having two beakers, one for putting in mixture and the other for the condensation mechanism, and a heating source.
- Take a distillation apparatus and pour the mixture in it. Make sure the distillation apparatus is clean.
- Start heating the mixture with the Bunsen burner flame
- Keep heating the mixture until it boils.
- Alcohol has B.P of 78.5C and water having a B.P of 100C, so we get vapors of the alcohol first.
- Alcohol vapors are condensed back to liquid by condensation mechanism.
Can we get 100% pure alcohol from the above process?
The quick answer to this question is no. The distillation process explained above is called “simple distillation.” It gives you satisfactory results when the mixture contains only two liquids, having boiling point differences of great margin. But it doesn’t mean that it will give you 100% results for two liquids. When the mixture is initially heated we get vapors of alcohol first with almost no contents of water, but as process goes on the temperature of mixture rises causes the water molecule also to evaporate.
Is there any way to get almost 100% results?
The answer here is yes. A process called “fractional distillation” can give very pure output. In fractional distillation, the process of evaporation and condensation is repeated till the final output (alcohol) becomes free of all undesirable contents. The process is a bit complicated from simple distillation, and one may need sophisticated equipment to carry out this process.
How to measure the purity of distilled Alcohol?
This is very simple, recall the basic formula of chemistry:
Density = Mass/Volume
We know the density (tabulated value) of pure alcohol. Feed the value of mass (Alcohol extracted during distillation process) and its volume into the above formula. Compare the result (Density) with the tabulated one.
Reference:
Sarton, George (1975). Introduction to the history of science. R. E. Krieger Pub. Co.. p. 145.
Holmyard, Eric John (1990). Alchemy. Courier Dover Publications. p. 53.
Chen, Zachary. “Modern Distillation”. Alcohol Aficionado: Spirits Ratings, Reviews, & History