Explanation of Hydrogen Production
Introduction to Hydrogen Production
Hydrogen fuel cells use hydrogen and oxygen in a chemical reaction with catalysts to produce electricity.
This power can be used in our homes and offices, electrical vehicles and even spaceships, such as in the NASA space shuttle using electrical power from hydrogen fuel cells and the resultant water for drinking!
Hydrogen gas can be used as a fuel having a high calorific value, burning with a hydrogen flame which is colorless and invisible.
There are many methods of producing hydrogen, the most popular at present being from the methane contained in natural gas, methane having one carbon atom and four atoms of hydrogen.
This is an article on the production of hydrogen and in particular from the use of renewable sources as opposed the more conventional methods. We begin by having a look at some current processes used to create hydrogen and then going on to examine biological methods.
Methods used to Produce Hydrogen
- Natural Gas
This is achieved by a process known as steam reformation where methane is subjected to steam injection to produce carbon monoxide and Hydrogen. The carbon monoxide is removed after which the hydrogen is isolated. The majority of hydrogen produced today is processed using this method
- Hydrogen Electrolysis
In this process water (H2O) is broken down into oxygen (O2) and Hydrogen gas (H2) This is achieved by passing an electric current through the water via an anode and cathode usually of platinum or stainless steel all contained within an electric cell.
Hydrogen Economy
Electrolysis is one of the most expensive methods of hydrogen production, as to achieve a worthwhile output of hydrogen excess electrical current must be passed through the cell, to increase potential energy.
- Biological
There are several methods of producing hydrogen gas from biological processes,
Fermentation
Dark fermentation entails the anaerobic fermenting of organic waste converting bacteria into hydrogen gas.
Photo fermentation uses light to break down the bacteria into fatty acids from which hydrogen is isolated and extracted.
Bio-hydrogen
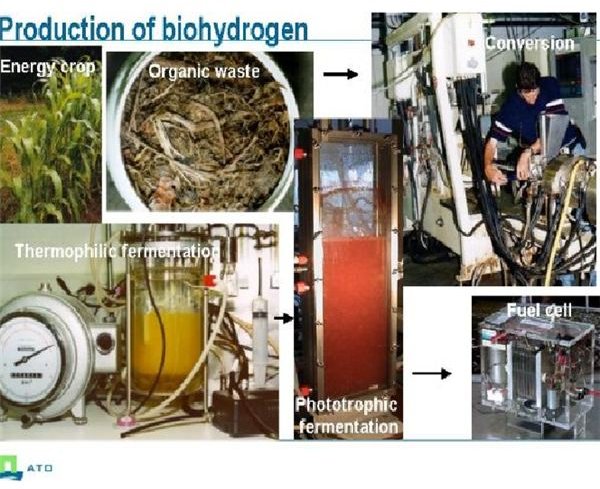
The hydrogen is produced in a bioreactor from various feed stocks including wastes and algae; the method using algae will be explored fully in the next section.
Production of Hydrogen from Micro-Algae
This method is similar to producing oil from microalgae (see my article on Algaoil from algae) where the algae is circulated through a photobioreactor. Here due to photosynthesis which it fixes CO2 from air producing sugars and fats whilst emitting oxygen.
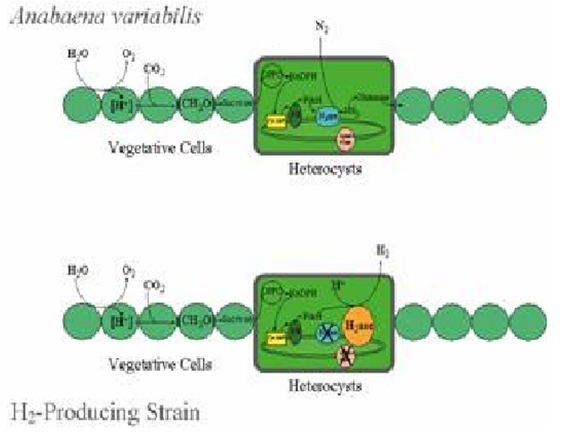
However, in previous research and development on different groups of microalgae, it was observed that the emission of oxygen would switch to hydrogen for no apparent reason. It was not until relatively recently that it was discovered to be due to hydrogenase; molecular hydrogen generated from biochemical energy.
This molecular hydrogenase is only present in certain groups of microalgae, being produced by the algae from absorbing biochemical energy through photosynthesis. It is inhibited by the presence of oxygen or excessive amounts of sulphur.
The process has now been modified to produce molecular hydrogen, through the elimination of oxygen and a reduction in sulphur in an innovative biotechnological process of microalgae in a photobioreactor.
The molecular hydrogen is further processed to produce hydrogen gas.
Images of Microalgae from (http://gcep.stanford.edu)
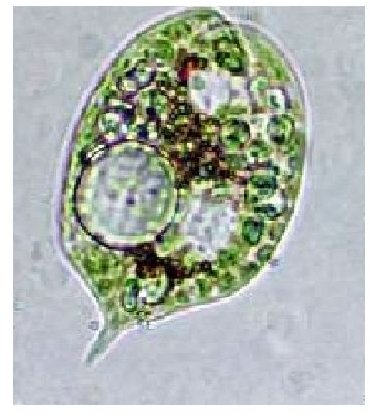

Internet Sites Visited
https://www.hydrogenassociation.org/
https://gcep.stanford.edu/images/factsheets/biohydrogen_generation2.jpg
https://www.napier.ac.uk/randkt/rktcentres/bfrc/PublishingImages/MF.bmp