Aluminum Food Safety Practices and Information about Aluminum Food Additives
The Need for Aluminum Food Safety Guidelines
The alarming reports about the presence of aluminum, not only in food as indirect additives, but also as contaminants in drinking water and in the air, and as material for cooking implements, gives rise to the consumers’ need for a definitive guide for aluminum food safety practices.
The problem that most consumers encounter when searching for one is that the guidelines available are those provided by the US Food and Drug Administration (FDA) for use of food manufacturers.
In view of this, we have gathered various data pertaining to aluminum, which could serve as a useful guide for food safety practices to reduce the risks of taking in large quantities of aluminum.
First, it is important to know the level of aluminum intakes considered safe by food experts.
The Provisional Tolerable Weekly Intakes (PTWI) for Aluminum as Recommended by the Joint Food and Agriculture Organization (JECFA)

The JECFA/World Health Organization Expert Committee on Food Additives made a re-assessment of their 2006 recommended aluminum food safety level. Said re-assessment had led to conclusive evidence that supported the lowering of its safety recommendation for PTWI, from 7mg/kg body weight to 1 mg/kg body weight. This includes additives that contain aluminum substances, although these are subject to further re-evaluation in the future.
The reassessment came amidst studies that revealed the widespread use and occurrence of aluminum for the production of food and of various products and occurring in the environment in the following structures and uses:
-
As buffering or neutralizing agents in the form of baking powder; (1) Aluminum Ammonium Sulfate: this can cause irritation if inhaled and ingested. Its decomposition is known to emit toxic fumes. (2) Aluminum Potassium Sulfate: considered as a nuisance dust.
-
As flavoring agents for beverages and candy; Aluminum Ammonium Sulfate
-
As anti-caking agents in the form of table salts and vanilla; Aluminum Calcium Silicate: considered as a nuisance dust.
-
As coatings for medicines, vitamins and dietary supplements; Aluminum nicotinate: applied heat can cause its decomposition which produces acrid smoke and fumes.
-
As firming agents for processed fish, vegetables and fruits; (1) Aluminum Sodium Sulfate, (2) Aluminum Ammonium Sulphate, (3_) Potassium Aluminum Sulphate and (4) Sodium Aluminum Sulphate._
-
As a food coloring additive; Sodium Aluminum Phosphate
-
As dusting agents in chewing gums; Magnesium Aluminum Silicate
-
As cooking implements in the form of aluminum pots and pans; anodized aluminum.
-
As raw materials for foil wraps;aluminum prepared into thin metal leaves.
-
As binder, emulsifier and stabilizer for food packaging materials; Aluminum Monostearate or stearic acid aluminum dihydroxide: heat can cause its decomposition, which produces acrid smoke and fumes.
-
As fumigants in the production of corn grits, brewer’s malts and brewer’s rice; Aluminum Phosphide: included in the EPA’s list of extremely hazardous substances, since it is highly poisonous through inhalations as well as flammable and dangerous when wet. FDA regulates its residue to only 1.01 ppm on processed foods and 0.1 ppm residue limitations in animal feeds.
-
As boiler-water additive for various uses; Aluminum Sodium Oxide also called NALCO 680, sodium aluminate, sodium aluminate oxide, sodium polyaluminate: corrosive materials, which can cause irritations to the eyes and mucous membranes.
-
As ingredients to glues or adhesives used for packaging pickle relish, pickles, potatoes and shrimp packs; Aluminum sulfate: moderately toxic and fumes are emitted upon decomposition.
-
As they may pervade in the air through small amounts released by coal-fueled power plants and incinerators; and
-
As they may occur in contaminated drinking water emanating from the EPA’s National Priorities List (NPL), which refers to sites considered as the most serious sources of hazardous wastes that pose threats to human health.
The Agency for Toxic Substances and Disease, National Center for Environmental Health, has likewise aired their concerns over the wide prevalence of said substance. Based on their Toxicological Profile for Aluminum, it would be best for consumers to take the following circumstances into consideration This is in view of the lowered PTWI recommended by JECFA as aluminum food safety level: Please proceed to the next page for more details.
Toxicological Profile for Aluminum

Aluminum is said to be the most widely available of all metals on the Earth’s surface, since it has the ability to combine with almost all forms of the elements, be it water, soil, rock, minerals or air.
- Due to its widespread use in food production, the average US adult consumes or ingests an estimated seven to nine milligrams (mg) of aluminum in a day.
Industrial workers have higher risks of over-exposure as they breathe-in elevated levels of aluminum dusts pervading their respective workplaces. Examples of these places are mining sites and factories that process aluminum metal and which are powered by coal-fueled plants and incinerators. Observance of workplace safety practices, such as the use of breathing masks and containment controls, can lessen, if not eliminate the health risks associated with aluminum dusts.
-
Communities near the EPA’s 1,678 NPL sites are areas located near industrial plants, which were found to have released wastes or spent washes that contain aluminum into the environment. The substance’s ability to combine with other elements had increased its natural occurrence in water, as it can quickly combine with oxygen.
-
The normal concentration of aluminum found in natural water is estimated at less than 0.1 mg/L, except in places where the water has been determined to be acidic.
-
In cities and urban areas, its concentrations tend to elevate up to 0.4 mg/L, as they undergo filtration or water treatment processes to render the water as potable.
-
The levels that pervade the atmosphere are estimated to range from 0.005 to 0.18 micrograms per cubic meter of air. Actual levels depend on the weather conditions, the location, and the concentration of industrial activities in the area.
-
Pollution that affects air quality comes in the form of suspended aluminum dusts or particulate matters. It is possible for dwellers in urban areas to breathe-in aluminum dusts measuring 0.4 µg/cu.m to 8.0 µg/cu.m, which could cause lung problems.
-
Human breast milk has a natural concentration of aluminum that ranges from 0.0092 to 0.049 mg/L. The reported average food safety levels in milk-based infant formula range from 0.058–0.15 mg/L, while the report for soy-based infant formula has placed the aluminum contents at higher levels of 0.46–0.93 mg/L.
-
As this element enters the body, it can go out quickly in the form of human solid wastes. Accordingly, aluminum components easily combine with the oxygen in water. Its intakes are rarely absorbed in the bloodstream, and in the rare occasion that it does, it will pass through the kidneys and exit by way of urine.
-
Those who have kidney problems are diagnosed to have high levels of aluminum in their bodies. A malfunctioning kidney may lead to bone or brain diseases, but there is still no conclusive evidence to prove that the Alzheimer’s disease are caused by high levels of aluminum found in impaired kidneys.
-
Children, however, are more susceptible to bone and brain diseases caused by the occurrence of aluminum in their digestive systems. Based on examinations of children diagnosed with bone diseases, their regular intakes of medicines containing aluminum substances prevent their bodies from absorbing phosphate nutrients, which are elemental for the development of healthy bones.
-
Adults who were found to have the highest exposure to the detriments of the substance are those who take drugs that contain aluminum, such as antacids and buffered aspirins, as a daily routine or for several times in a week.
All these factors and circumstances should be considered when increasing one’s exposure to the various substances by way of food additives. Although the aluminum that naturally occurs in the environment is generally considered harmless, the measure of exposure as one goes beyond the aluminum food safety level, are usually determined by way of fecal and urine analysis. These tests are often required during medical examinations and diagnosis, as symptoms of certain diseases or disorders begin to manifest.
Measuring bone aluminum, however, is not readily available in medical clinics, since the examination required is by way of bone biopsy. This type of medical test requires the extraction of tissue samples for examination by way of microscopic view, in order to determine the cause of a disorder or disease.
What are the Current Federal Regulations for Aluminum as a Food Additive or Contaminant?
The Environmental Protection Agency (EPA)
The EPA has recommended that the amount of 0.05–0.2 milligrams per liter (mg/L) of aluminum in drinking water be observed as a Secondary Maximum Contaminant Level (SMCL). This is in view of EPA’s findings that toxic contaminants in different forms are present in about 606 NPL sites.
These areas are now called Superfund sites, in view of the CERCLA law which mandated their clean-ups. They have been ascertained as highly contaminated with hazardous substances, which include aluminum. In order to determine if you are living near established Superfund site, you can check it out at this EPA webpage entitled “Superfund Sites Where You Live”.
Food and Drug Administration
As a food additive, the FDA has classified certain aluminum compounds used in food, medicines, health supplements and packaging materials as GRAS (Generally Recognized as Safe).
The uses of aluminum in manufacturing processes are said be regulated by the FDA under the provisions of the Good Manufacturing Practice (GNP) Regulations. However, a close examination of the regulations disclosed that these are general provisions that pertain to process validation, equipment verification, complaint handling, personnel qualifications, record keeping, cleanliness, and sanitation as guidelines to be observed by manufacturers.
In addition, the regulations appear as open-ended statements that give the manufacturer’s flexibility for compliance, in the way they deem it as best practice, albeit using up-to-date technologies that will prevent contamination and/or errors.
Labeling
The FDA requires all drug manufacturers to maintain no more than 25 micrograms per liter and the label and package must state the amount of aluminum used. The following warnings should be carried in the warning section of the labels and package inserts;
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
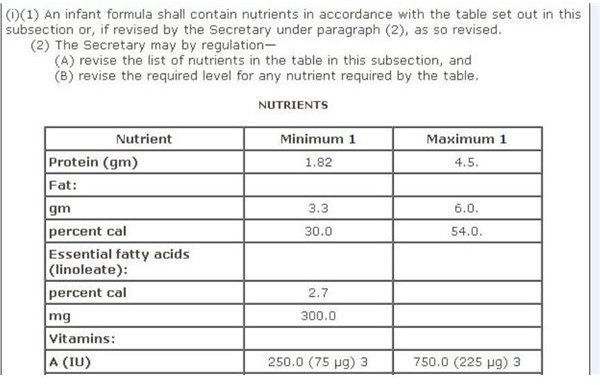
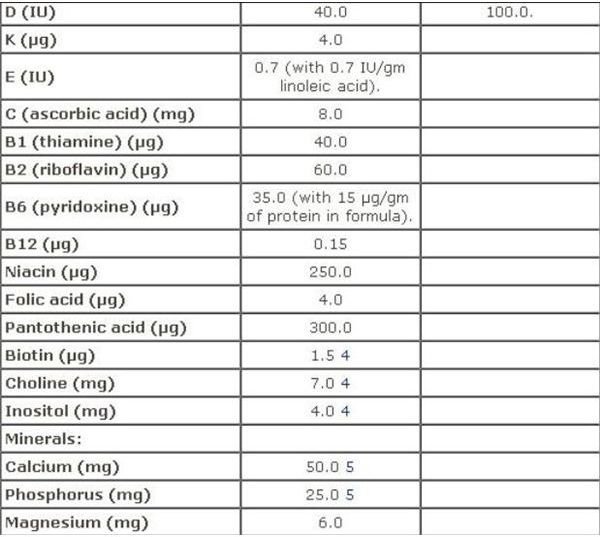
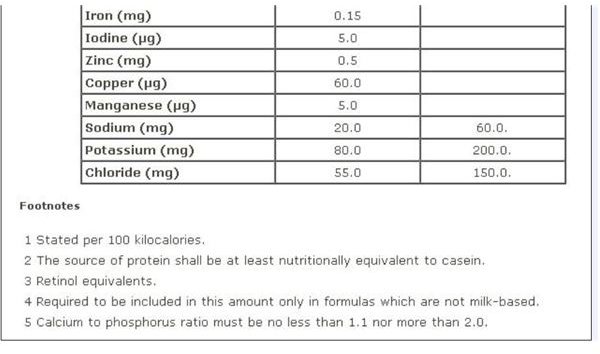
Infant Formula
FDA regulations for infant formula are contained in Sec. 412 [21 USC §350a] Requirements for Infant Formulas, which furnishes a Table of Nutrients for infant-based formula. A screen-shot image of this recommendation is provided on your left, which you can view in full on the aforementioned webpage.
Food Additives
Consumers who would be interested to know about the different aluminum food safety additives classified as Generally Recognized as Safe , can find available information in FDA’s webpage entitled “Everything Added to Food in the United States (EAFUS)”, from where you can launch the database containing said information.
Other Food Safety Recommendations
-
Be wary of aluminum pots, pans, and foil wraps as they tend to leach. Recommended alternatives to these cooking implements are cast iron, stainless steel, ceramic, glass, silver and wooden utensils.
-
Avoid storing highly acidic food such as tomatoes, rhubarb, cabbage, and soft types of fruits in foil wraps and containers as their juices may combine with the aluminum substance while kept in storage for a long time.
-
Avoid aluminum beer and soda cans, particularly those that contain citric acid, since this substance can combine and increase the absorption efficiency of aluminum.
-
In relation to this, some researches disclosed that other acid forming foods like coffee, black and green tea, spinach, cheese, meats, cabbage, cucumbers, tomatoes, turnips, and radishes promote aluminum absorption in the body.
-
Aside from antacids, check other over-the-counter drugs such as painkillers, anti-inflammatory, aspirins, and anti-diarrheas for aluminum content.
-
Limit intakes of muffins, waffles, and pancakes due to their high levels of aluminum additives.
-
Avoid acidic diets and those that limit the intakes of phosphate as this can promote rapid bone degeneration due to the presence of aluminum.
These are only warnings about aluminum and food safety practices. Consumers should be aware that personal care products like deodorants, toothpaste, shampoos, and douche preparations, also contain aluminum as active ingredients; hence increasing risks of aluminum exposure.
Reference Materials and Image Credit Section:
References:
- https://www.atsdr.cdc.gov/phs/phs.asp?id=1076&tid=34
- https://www.cfs.gov.hk/english/programme/programme_rafs/files/Guidelines_on_the_use_of_Al_additives_e.pdf
- https://www.naturalpedia.com/A/Aluminum-food.html
- Food Additive Handbook By Richard J. Lewis (Sr.)https://books.google.com.ph/books?id=nC7OGhzZn5YC&pg=PA134&lpg=PA134&dq=EPA+on+aluminum+food+additives&source=bl&ots=i-nLjkBFS4&sig=GaYnngTiZEwSHDjhzgryTqx8X4I&hl=en&ei=w9n_TKSvFMLyrQeNoYidCA&sa=X&oi=book_result&ct=result&resnum=9&ved=0CFIQ6AEwCA#v=onepage&q&f=false
Image Credits: