Ethanol Production Process: Dry Mill Process
Introduction
The world is presently confronted with two major crises – Global warming and rising prices of non-renewable fuels. However, both of these problems have one common solution – an alternate renewable fuel source. Global warming is one prime concern that is pushing researchers and scientists around the world to look for a better and more environmentally friendly fuel source. Ethanol is one such alternative fuel source that has been receiving great recognition because of its several benefits. Ethanol has also been termed as a prospective transportation fuel source by many researchers around the world. Though there are many pros and cons to it, ethanol is commonly used as a fuel additive in several countries.
How is Ethanol Produced?
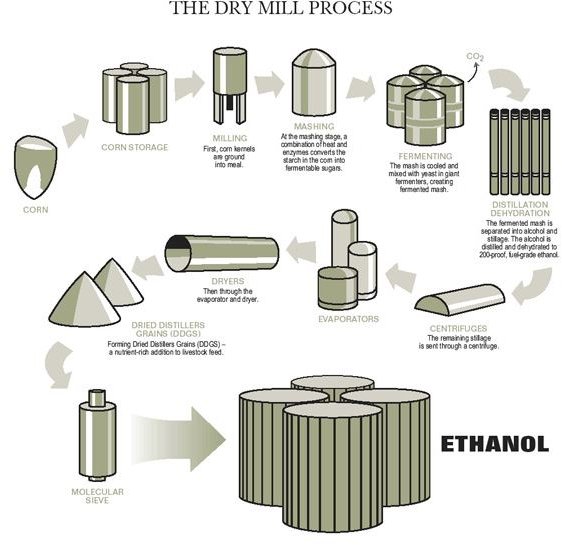
Ethanol is a type of biofuel, which means that it is made by naturally available materials. Ethanol is generally made by a process known as “The Dry Mill Process.” The main sources of ethanol are starchy crops such as corn, barley, and sorghum, sweet crops such as citrus, sugar beets, and sugar cane, and, even plants and tress such as switch grass and wood pulp.
For making ethanol, any of the above mentioned sources are first ground into small particles using special machinery. This process is known as milling. The starch particles thus acquired are further converted into powdered form. Once this is done, water is added to the mixture to make a fine mash. A special type of enzyme (alpha-amylase) is then added to the mixture which further converts it into tiny little particles. This whole process takes place in a sealed container and is known as the mashing stage. Water makes the starch liquefy and reduce bacteria. The mixture is then heated which converts the starch into fermentable sugar completely.
The mixture is then transferred to another apparatus wherein it is cooled and yet another enzyme is added to it. The added enzyme completely converts liquid starch to sugar and prepares the mixture for fermentation. Yeast is added to the mixture and for 48 hours the mixture is churned. This is the process wherein the sugar changes to ethanol and the natural carbon dioxide is released from the mixture. The mash at the end of 48 hours contains 10% ethanol.
To extract the ethanol from the mash, the mixture is again heated up at high temperature. The ethanol from the mixture vaporizes and rises to the top of the apparatus from where it is collected. The collected vaporize ethanol is cooled and then condensed to liquid form. However, this acquired mixture is not of the purest form and thus needs to be purified. The major content of this is water, and thus extra amount of H2O is removed and the remaining liquid is purified to get the pure and usable ethanol which is about 200 proof. However this ethanol is not of the portable form. To make it transportable, a small amount of gasoline is added to the ethanol, approximately 2-5%. This process is known as denaturing and is a prime requirement for all fuel grade ethanol. The ethanol thus produced can be transported and used as fuel for automobiles.
This is not all. The left over from the process such as waste, grains and, carbon dioxide can also be used for various other purposes. The corn residue or grains is a nutritious livestock feed and the carbon dioxide released is used in making carbonated beverages.
Thus, ethanol serves as an ideal source of renewable fuel, which not only suits the present world situation but also has a great future. However, the present technology still needs to be pushed harder to a point where ethanol can be used as a fuel substitute and not as a fuel additive.