Alembics - Old Technology for Distillation
History and Use
The history of distillation in general dates back to 200 – 300 A.D. It is a distillation process where there is a separation of more than one liquid of different boiling points into a more pure form. The purpose this serves is to separate undesirable parts of a liquid and obtain the more preferred component. It is often used in alcohol production (i.e., at a distillery), with crude oil, for the production of a more usable fuel, and for the desalinization of seawater into freshwater.
What is an alembic? Alembics were basically distillation equipment used for these processes. All it took was boiling the liquid in one container and then funneling the liquid vapor, usually via gravity, into another container where it was cooled, condensed, and brought back to its liquid state. The resulting product of distillation was then used for whatever corresponding function it was intended for. Again, the purpose of this was to boil out the unwanted impurities in the original raw oil.
In 850 A.D. the Muslims perfected the process. Their alembic equipment consisted of three components: a flask in the shape of a gourd that held the intended crude petroleum product to be distilled, also known as the curibit; a condenser device that captured the distillate or vapors that rose up from the crude oil; and on the receiving end of it all, the collection vessel that received the distilled oil from the spouted curibit.
The end result was called “White Naptha” or in Muslim terms, “naft abyad”, which was used in lamp oil.
Their process was pretty much similar to current methods, except with smaller volumes and without the continuous distillation towers that we use today. The Arabs used, in their terms, an “al-inbiq” which thus coined the English variant of the term, “alembic.” Though spelled either way, it sounds the same.
Distilling with an Alembic
The components of which kerosene is distilled from petroleum chemicals are found below the surface of the ground. These would include the rocks, water, oil, and other contaminants found in the surrounding porous layers of carbonate rock and sandstone. Basically, kerosene is extracted from the crude oil itself. Multiple and fractional distillation methods are needed to provide the alembic distillate kerosene in its pure form.
Due to the repeated distillation processes required to reach the desired results, kerosene production isn’t entirely efficient. Leftover unconverted hydrocarbons need to be sent through the distillation process again and again to achieve the pure form of this resource.
There are sometimes resulting byproducts in addition to just the kerosene. Paraffin wax is another usable waste product as the result of the distillation process, as well as other oil related lubricants.
Fractional Distillation of Kerosene
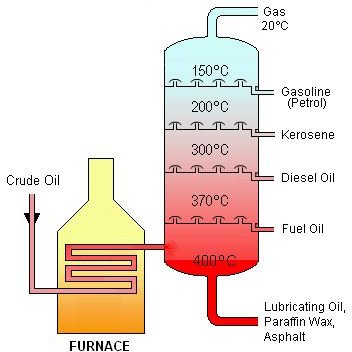
The basic method of how crude oil is distilled involves a tank or reservoir and a header column or fractionating tower into which the crude is delivered and “boiled off.” As shown in the diagram below (click image to enlarge), as the distillate rises and cools in the distillation column, certain areas of the distillate are sectioned off depending on their corresponding cooling points. For example, regular oil for lubrication that’s cooled around 400 degrees Celsius is sectioned off and extracted. Notice that the highest point is reserved for petroleum gas. It is quite fascinating that each petroleum product distillate has its own designation, which is defined by its cooling temperature.
The Future of Kerosene
Applications are being are being considered to replace current jet diesel fuel with high grade kerosene fuel, also known as JP-8. To prevent diesel fuel from “gelling” in cold weather, kerosene can be used as an additive in low sulfur-containing diesel fuels.
Another awesome outcome of kerosene is one of a low-misting variety. When used in airline fuels, this can decrease the likelihood of explosions. So, statistically speaking, it can even made the safest form of travel even safer!
Source Links
University of Delaware Library: From Liquid to Vapor and Back: Origins
History of Alcohol Distillation
Enotes: How Products are Made - Kerosene
